FDA-Cleared Autism Assessment Technology
Diagnose Earlier.
Treat Sooner.
Measure Outcomes.
FDA-cleared eye-tracking technology using visual attention biomarkers for autism to support early diagnosis and measurable treatment outcomes—starting as young as 16 months.
Study Locations






Join the EarliPoint® Network
Answer a few quick questions to see if you qualify for low-cost access to EarliPoint® and opportunities to join the EarliPoint® Network. Takes ~2–3 minutes.
How It Works

Quick and Easy Administration
A trained behavior technician—or any designated trained team member—can easily administer the assessment using a portable tablet.

Eye Gaze Capture
Children watch a video while the device unobtrusively captures eye gaze data. Behind the scenes, proprietary algorithms analyze patterns of visual attention—biometric markers of social and cognitive development.

Actionable Insights
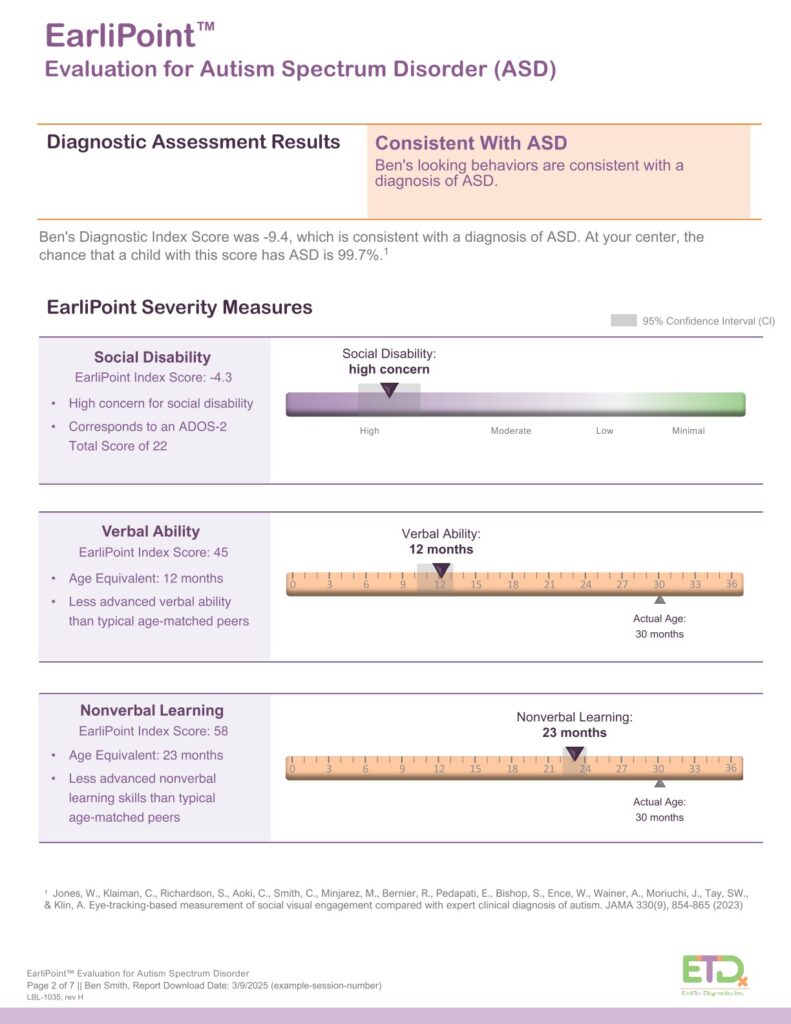
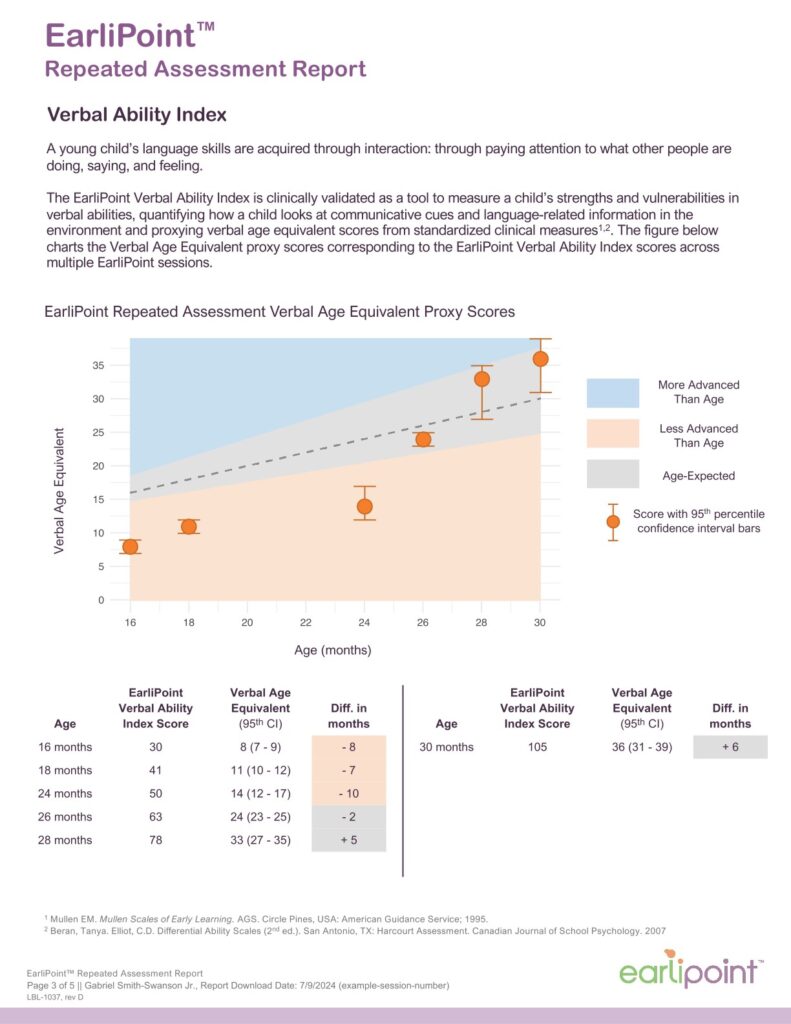
The system generates a detailed report showing the child’s scores across three clinically aligned indices: language, cognition, and social engagement. These insights support early detection and help track progress over time.
Supporting Timely, Informed Care
Same-day results help guide discussions with families and care teams.
The EarliPoint System device is indicated for use as a tool to aid qualified clinicians in the diagnosis and assessment of Autism Spectrum Disorder (ASD) in children ages 16 months through 30 months who are at risk based on concerns identified by a parent, caregiver, or healthcare provider (Not for direct consumer purchase; clinician use only).
Benefits for Providers
A Smarter Way to Grow Your Practice
Transform your practice with FDA-cleared diagnostic aid that provides outcome measurements and builds stronger relationships with families.
Attract New Patients
Offer FDA-cleared diagnostics to families—removing barriers to early assessment and differentiating your practice in the community.
Track Treatment Effectiveness
Communicate Outcomes with Confidence
Earn the confidence of parents, payors, and educators with transparent, easy-to-read reports that highlight progress clearly and consistently.
Proven Science
Decades of research. Breakthrough results.
The EarliPoint® System is FDA-cleared to provide visual-attention measurements that aid qualified clinicians in the diagnosis and assessment of Autism Spectrum Disorder, alongside established diagnostic tools.
Our research foundation includes:
- Clinical Validation — Two large-scale, double-blind clinical studies
- Peer Review — Findings published in the Journal of the American Medical Association (JAMA)
- Academic Excellence — Research conducted in collaboration with Yale University and Emory University

DATE: September 5, 2023
Eye-Tracking-Based Measurement of Social Visual Engagement Compared with Expert Clinical Diagnosis of Autism.

DATE: September 5, 2023
Development and Replication of Objective Measurements of Social Visual Engagement to Aid in Early Diagnosis and Assessment of Autism.
Frequently Asked Questions
EarliPoint Health is a healthcare technology company transforming access to autism care through research-based clinical innovation. Founded on decades of scientific discovery, the company develops tools that empower clinicians with objective insights into a child’s development—enabling earlier understanding, more informed intervention, and measurable outcomes that may improve lifelong trajectories.
The EarliPoint System is an FDA-cleared medical device indicated for use as a tool to aid qualified clinicians in the diagnosis and assessment of Autism Spectrum Disorder (ASD) in children 16 to 30 months old who are at risk based on concerns shared by a parent, caregiver, or healthcare provider.
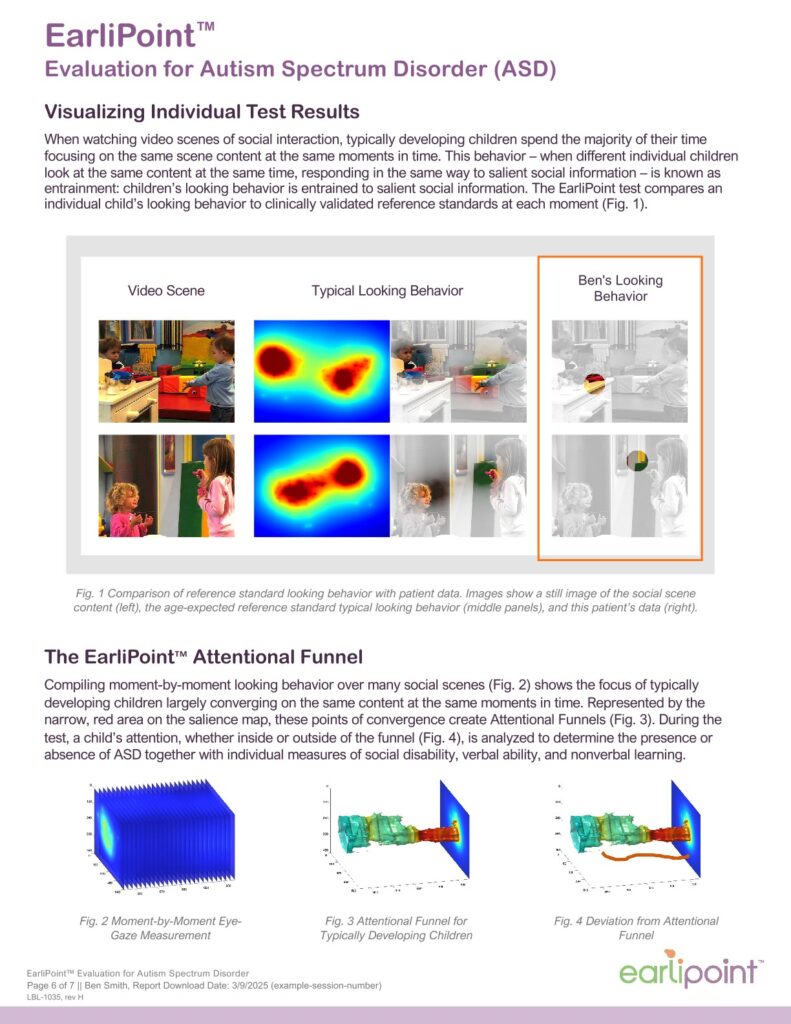
Using advanced eye-tracking technology, the system measures how a child visually engages with social and non-social scenes. These data provide objective, quantifiable information that complements a clinician’s observation and judgment during the diagnostic process.
During an evaluation, a child watches video scenes featuring social interactions. Eye-tracking sensors record where and how long the child looks at different parts of each scene. The resulting visual-attention data are analyzed by algorithms developed through years of developmental research, providing objective measures that help clinicians assess patterns associated with ASD.
The EarliPoint System is FDA-cleared. FDA clearance means the device has successfully demonstrated substantial equivalence to a legally marketed device in terms of safety and effectiveness under a 510(k) submission. This clearance confirms that the EarliPoint System meets the FDA’s rigorous standards for use as a medical device to aid clinicians in the diagnosis and assessment of ASD.
The EarliPoint System is designed for clinical use only and is not available for direct consumer purchase. Data collection can be completed by anyone trained to use the system, such as clinical staff or technicians. However, interpretation of results must be performed by qualified clinicians trained to diagnose ASD according to state guidelines.
The system is indicated for children 16 to 30 months old who are at risk for ASD based on concerns raised by a parent, caregiver, or healthcare provider.
No. The EarliPoint System does not make a diagnosis on its own. It serves as a tool to aid qualified clinicians by providing objective data that support their diagnostic assessments. The final diagnosis and care decisions remain with the clinician.
Early evaluation creates opportunities for timely support. Research shows that when developmental differences are identified before age three, children are more likely to benefit from intervention during a period of heightened brain plasticity—when learning, language, and social skills are rapidly developing.
Autism affects roughly 1 in 31 children. Yet, the average age of diagnosis in the U.S. is over 4 years old, well past the optimal window for early intervention. Long waitlists, subjective tools, and limited access to qualified providers delay evaluation—especially for rural and underserved families.
EarliPoint Health’s work addresses these challenges by offering objective, standardized tools that help clinicians identify developmental differences sooner and with greater consistency.
Traditional diagnostic tools rely heavily on observation and parent reporting, which can be subjective and time-intensive. EarliPoint Health introduces objective, technology-based measures that complement clinical judgment—helping clinicians reach insights faster and more consistently while maintaining diagnostic rigor.
Why We Do This

Beverly Johnson, MHA, MS
At Easterseals Florida, we are driven by possibility. We believe that the earlier children receive services tailored to their unique needs, the greater their long-term success. Early diagnosis not only provides families with answers—it sets them on the right path, connecting them with critical support and services when they can make the greatest impact. EarliPoint empowers us to do just that.

Abigail Ley, MD
Pediatric Neurologist

Sondra Marshall, PhD
Clinic Director, PEDAL
In our 30,000-square-mile catchment area of Central, Eastern, and Southern Oregon, EarliPoint has been transformative in improving access to timely autism diagnosis. By placing devices in primary care clinics, we reduce travel burdens for families and can complete evaluations remotely, combining the objective EarliPoint data with developmental screenings and clinical interaction to strengthen diagnostic confidence. This approach has significantly shortened wait times, empowered providers, and given families earlier access to answers and treatment. Families who come to our clinic have expressed increased confidence and acceptance of the differential diagnosis when they see the EarliPoint results that match our neurodevelopmental testing.